Resin recycling: New catalysts can recover material from epoxy compounds that are virtually impossible to recover.
Epoxy resin is experiencing a period of extraordinary success both in the industrial sector and in various independent business downturns, which are reflected to varying degrees on social media. Epoxy compounds are hiding in plain sight in most places people visit: they can be found on floors, in containers, in car bodies, in electronic devices, and even inside shoes and clothes.
Despite their widespread use, these compounds still pose challenges in terms of recycling and responsible disposal . In fact, handling these materials has proven to be extremely difficult. Therefore, at the end of their useful life, items containing epoxy resin are most often thrown into landfills unchanged.
However, for the first time, a group of scientists has succeeded in effectively extracting material from epoxy products: to do this, the researchers developed a new solid catalyst that can also be used for other types of plastic that are very difficult to recycle.
Can epoxy resin be recycled?
Epoxy compounds They are used in a wide range of production: in electronics due to their insulating properties, in construction due to their strength and, as a very effective impregnating material, when laying composite materials such as carbon fiber and fiberglass.
However, the long history of epoxy resins, which began in 1939 with a German-Swiss dual patent, is fraught with some problems. The environmental costs are irrelevant, both in terms of pollution and the carbon footprint of a key production sector. For example, a 2019 study found that epoxy resin synthesis accounts for more than 84% of the total carbon footprint of the automotive industry when considered over its entire life cycle.
Epoxy compounds are also very difficult to process after use, as are all products containing them – from car bodies to wind turbine blades, from floors to ship and yacht hulls. Unlike thermoplastics such as polyethylene terephthalate, their epoxy composites are virtually unsuitable for mechanical recycling due to the deterioration of the material properties, which, once cured, cannot be re-melted and re-formed.
How do you explain Xiong Jie Jin , Associate Professor at the University of Tokyo,
“To break down fiber-reinforced plastics, such as those used in aircraft components, you need high temperatures, above 500 degrees Celsius, or strong acidic or alkaline conditions. In addition to the energy costs, these harsh conditions can damage the fibers and the object you are trying to repair.”
Together with his teacher Kyoko Nozaki and their research group, Jin developed a new solid catalyst that can decompose epoxy compounds into carbon fibers, glass fibers, and phenolic compounds. The study was published in the journal Nature Communications .

Solid catalysts for the processing of resins and composite materials
To overcome the difficulties associated with the high cost of proven recycling methods, Japanese researchers have used a relatively new process called catalytic hydrogenolysis , which uses hydrogen as a reactant to cause the cleavage of carbon-oxygen bonds present in epoxy resins.
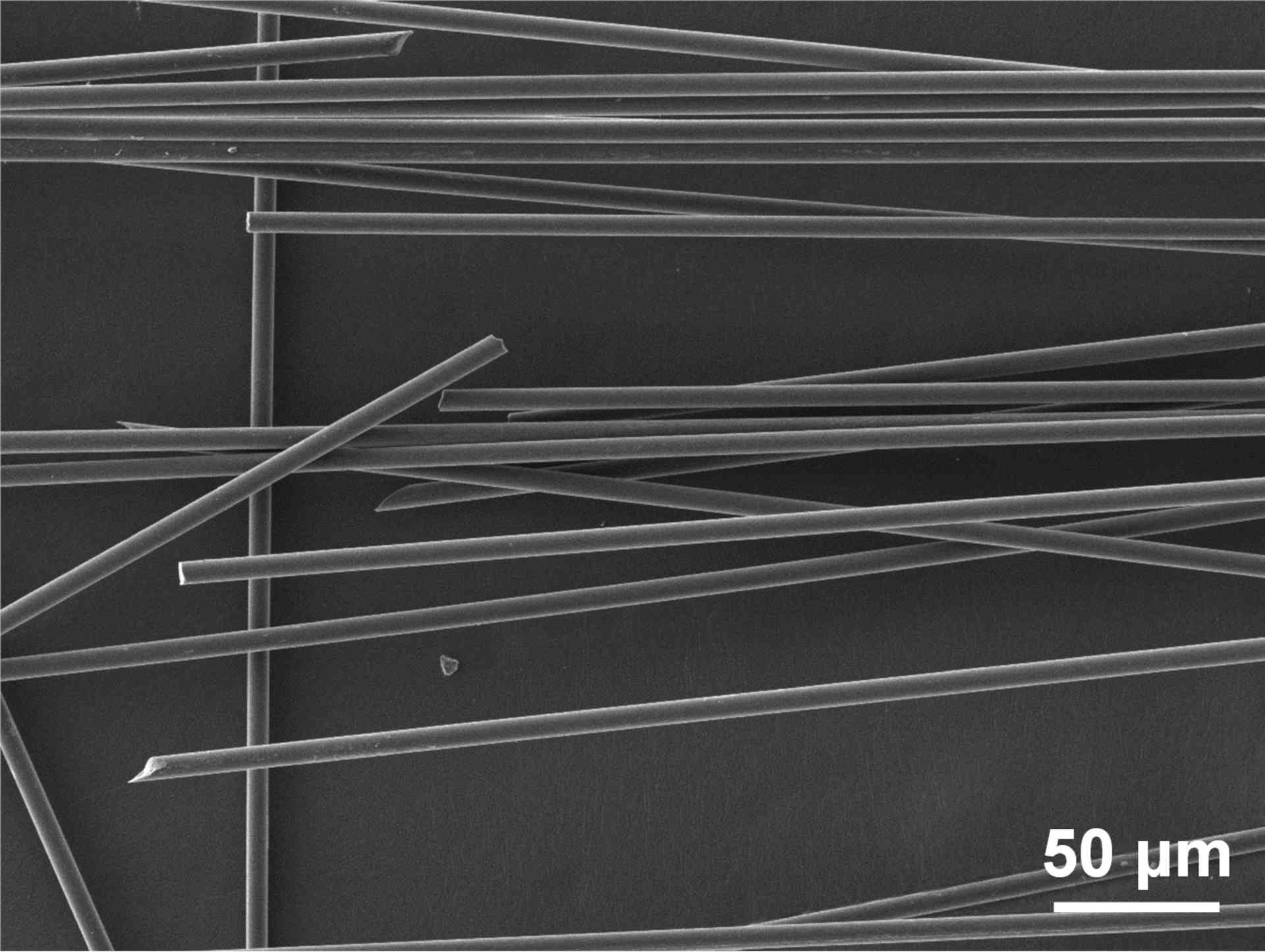
As Jean explains,
“Catalytic hydrolysis shows promising results, but existing catalysts cannot be reused because they dissolve in the solvent where the epoxy resin decomposes. Therefore, we created a new solid catalyst that is easy to recover and reuse.”
The new catalyst is called “bimetallic” because it uses two metals, nickel and palladium , which work together to mediate the reaction between the epoxy resin and hydrogen gas.
The reaction temperature of the new process is around 180 degrees : as the researchers explain, the energy requirement is much lower than that required to create conditions at 500 degrees. In addition, lower temperatures will allow the materials to be completely recovered , which opens up real prospects for their reuse.

New perspectives for epoxy compounds and plastics
This new solid catalyst has shown very promising results: in addition to being effective in the challenging task of regenerating epoxy materials, it has proven to be robust and potentially suitable for other applications. As Jin explains,
“We were pleased to see the experimental results, which were completely in line with our expectations of how this process would work, but we were particularly surprised to realize that the catalyst could be reused at least five times without any loss in performance.”
In addition, the professor explained, modified versions of this catalyst, being an effective catalyst for the cleavage of carbon-oxygen bonds, can also be used for other plastic materials , which in many cases contain such bonds.
However, before moving on to recycling other materials, the team wants to explore new methods and materials that can be applied to the new system to improve it and make it a viable option at a commercial level as well .
“While our catalyst does not require such high temperatures, there is still room to improve the environmental impact of the solvents we use. We also want to reduce costs by finding catalysts that do not contain precious metals such as palladium. The amount of recycled material from the various epoxy compounds could also be increased, thereby reducing the environmental costs of this very versatile and useful plastic .